what change would not result in a shift of the equilibrium position to the left?
| EQUILIBRIUM CONSTANTS and LE CHATELIER'S PRINCIPLE This folio looks at the relationship between equilibrium constants and Le Chatelier's Principle. Students often become confused almost how it is possible for the position of equilibrium to change as you change the conditions of a reaction, although the equilibrium constant may remain the aforementioned. Exist warned that this page assumes a good understanding of Le Chatelier'due south Principle and how to write expressions for equilibrium constants. | |||||||
| Important:If y'all aren't happy nearly the basics of equilibrium, explore the equilibrium menu before you waste your time on this folio. This folio should just be read when yous are confident about everything else to do with equilibria. | |||||||
| Changing concentrations The facts Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. The only thing that changes an equilibrium constant is a alter of temperature. The position of equilibrium is changed if you change the concentration of something present in the mixture. According to Le Chatelier'due south Principle, the position of equilibrium moves in such a fashion equally to tend to undo the change that you accept fabricated. Suppose you have an equilibrium established betwixt four substances A, B, C and D. According to Le Chatelier's Principle, if you decrease the concentration of C, for example, the position of equilibrium will move to the correct to increase the concentration again. | |||||||
| Annotation:The reason for choosing an equation with "2B" volition become clearer when I deal with the effect of force per unit area further downward the page. | |||||||
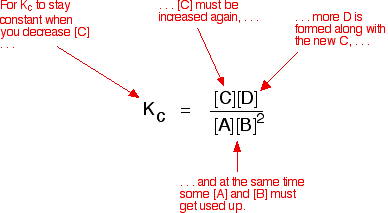
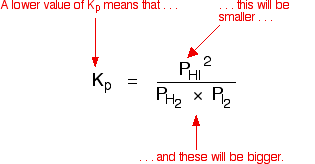
| Explanation in terms of the constancy of the equilibrium constant The equilibrium constant, Thouc for this reaction looks like this: If you lot have moved the position of the equilibrium to the right (and so increased the amount of C and D), why hasn't the equilibrium constant increased? This is actually the wrong question to ask! We need to look at information technology the other fashion circular. Let's assume that the equilibrium constant mustn't modify if you decrease the concentration of C - because equilibrium constants are abiding at constant temperature. Why does the position of equilibrium move as it does? If you decrease the concentration of C, the top of the Kc expression gets smaller. That would change the value of Gc. In lodge for that non to happen, the concentrations of C and D will have to increase again, and those of A and B must decrease. That happens until a new rest is reached when the value of the equilibrium constant expression reverts to what it was earlier. The position of equilibrium moves - non because Le Chatelier says it must - just because of the need to keep a constant value for the equilibrium constant. If you subtract the concentration of C: Irresolute pressure This simply applies to systems involving at least 1 gas. The facts Equilibrium constants aren't changed if yous change the pressure of the system. The merely thing that changes an equilibrium constant is a change of temperature. The position of equilibrium may be inverse if you alter the pressure. According to Le Chatelier's Principle, the position of equilibrium moves in such a way as to tend to undo the change that you have made. That means that if you increase the pressure, the position of equilibrium will move in such a way as to decrease the pressure over again - if that is possible. It can exercise this by favouring the reaction which produces the fewer molecules. If there are the same number of molecules on each side of the equation, then a alter of pressure makes no difference to the position of equilibrium. Caption Where in that location are different numbers of molecules on each side of the equation Let'due south expect at the aforementioned equilibrium nosotros've used earlier. This 1 would be affected past pressure because there are three molecules on the left simply only 2 on the right. An increase in pressure would motion the position of equilibrium to the right. Because this is an all-gas equilibriium, it is much easier to use Chiliadp: Again, it is easy to suppose that, because the position of equilibrium will move to the right if you increase the force per unit area, 1000p volition increment also. Not and then! To empathise why, you need to modify the One thousandp expression. Think the human relationship betwixt partial pressure, mole fraction and full pressure? | |||||||
| Note:If you aren't happy with this, read the beginning of the folio nearly Kp earlier you go on. Employ the Dorsum button on your browser to return to this page. | |||||||
| Replacing all the fractional pressure terms past mole fractions and total force per unit area gives you this: If yous sort this out, most of the "P"s abolish out - only one is left at the bottom of the expression. Now, think that Kp has got to stay abiding because the temperature is unchanged. How can that happen if you increase P? To recoup, yous would have to increment the terms on the top, 10C and xD, and subtract the terms on the lesser, xA and xB. Increasing the terms on the top means that you take increased the mole fractions of the molecules on the right-paw side. Decreasing the terms on the bottom means that y'all accept decreased the mole fractions of the molecules on the left. That is some other manner of proverb that the position of equilibrium has moved to the right - exactly what Le Chatelier'southward Principle predicts. The position of equilibrium moves so that the value of 1000p is kept abiding. Where in that location are the aforementioned numbers of molecules on each side of the equation In this instance, the position of equilibrium isn't affected by a change of pressure. Why not? Let'south become through the same process equally before: Substituting mole fractions and full pressure level: . . . and cancelling out as far every bit possible: In that location isn't a unmarried "P" left in the expression. Changing the pressure level can't make whatsoever departure to the Kp expression. The position of equilibrium doesn't demand to move to go on Mp constant. Irresolute temperature The facts Equilibrium constants are changed if you alter the temperature of the system. Grandc or Kp are constant at abiding temperature, but they vary as the temperature changes. Expect at the equilibrium involving hydrogen, iodine and hydrogen iodide: The Gp expression is: Two values for Kp are:
Yous can see that as the temperature increases, the value of Kp falls. | |||||||
| Notation:Y'all might possibly be wondering what the units of Yardp are. This particular example was chosen because in this case, Kp doesn't have whatever units. Information technology is just a number. The units for equilibrium constants vary from case to case. It is much easier to empathize this from a book than from a lot of maths on screen. You volition detect this explained in my chemistry calculations volume. | |||||||
| This is typical of what happens with any equilibrium where the forrad reaction is exothermic. Increasing the temperature decreases the value of the equilibrium abiding. Where the forrard reaction is endothermic, increasing the temperature increases the value of the equilibrium abiding. | |||||||
| Annotation:Any explanation for this needs knowledge beyond the telescopic of whatever Great britain A level (or equivalent) syllabus. | |||||||
| The position of equilibrium too changes if you alter the temperature. Co-ordinate to Le Chatelier's Principle, the position of equilibrium moves in such a way equally to tend to undo the change that you have made. If you increase the temperature, the position of equilibrium will move in such a way every bit to reduce the temperature again. It will practice that by favouring the reaction which absorbs heat. In the equilibrium nosotros've just looked at, that will exist the back reaction because the forwards reaction is exothermic. So, according to Le Chatelier'due south Principle the position of equilibrium volition move to the left. Less hydrogen iodide volition be formed, and the equilibrium mixture will contain more unreacted hydrogen and iodine. That is entirely consistent with a fall in the value of the equilibrium constant. Adding a goad The facts Equilibrium constants aren't changed if you add (or change) a catalyst. The only thing that changes an equilibrium abiding is a modify of temperature. The position of equilibrium is non changed if you add (or change) a catalyst. Caption A catalyst speeds upwardly both the forward and dorsum reactions past exactly the same amount. Dynamic equilibrium is established when the rates of the forward and back reactions become equal. If a catalyst speeds up both reactions to the same extent, and then they will remain equal without any need for a shift in position of equilibrium. | |||||||
| Note:If you know virtually the Arrhenius equation, it isn't too difficult to apply it to show that the ratio of the rate constants for the forward and dorsum reactions isn't affected by adding a catalyst. Although the activation energies for the two reactions alter when you lot add a catalyst, they both modify past the same corporeality. I'g non going to do this bit of algebra, considering it would never exist asked at this level (United kingdom A level or equivalent). | |||||||
| Questions to test your understanding If this is the commencement prepare of questions yous have washed, delight read the introductory page before y'all get-go. You volition need to use the BACK Button on your browser to come back here subsequently. questions on equilibrium constants and Le Chatelier's Principle answers
To the equilibrium menu . . . To the Concrete Chemistry menu . . . To Main Carte du jour . . . © Jim Clark 2002 (final modified March 2021) | |||||||
richmondtamet1951.blogspot.com
Source: https://www.chemguide.co.uk/physical/equilibria/change.html
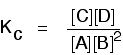
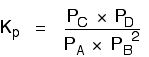

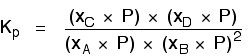
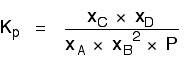
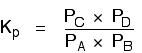
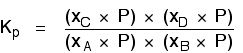
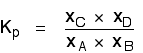
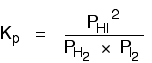
0 Response to "what change would not result in a shift of the equilibrium position to the left?"
Post a Comment